-40%
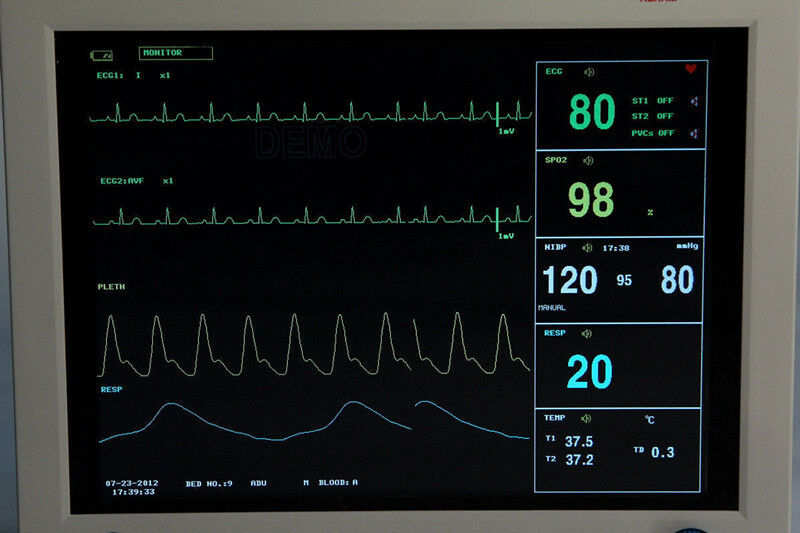
ICU CCU CONTEC CMS8000 Patient Monitor 6 parameter 12.1 Inch Vital Signs Monitor
$ 316.27
- Description
- Size Guide
Description
Ship from Illinois,united states. 2-5 days arrival.Tips:
USA Buyer:
ship from USA warehouse
. Fast delivery (2-5 days) ;
Other Buyer :
ship from China Warehouse
. 1-6 weeks delivery
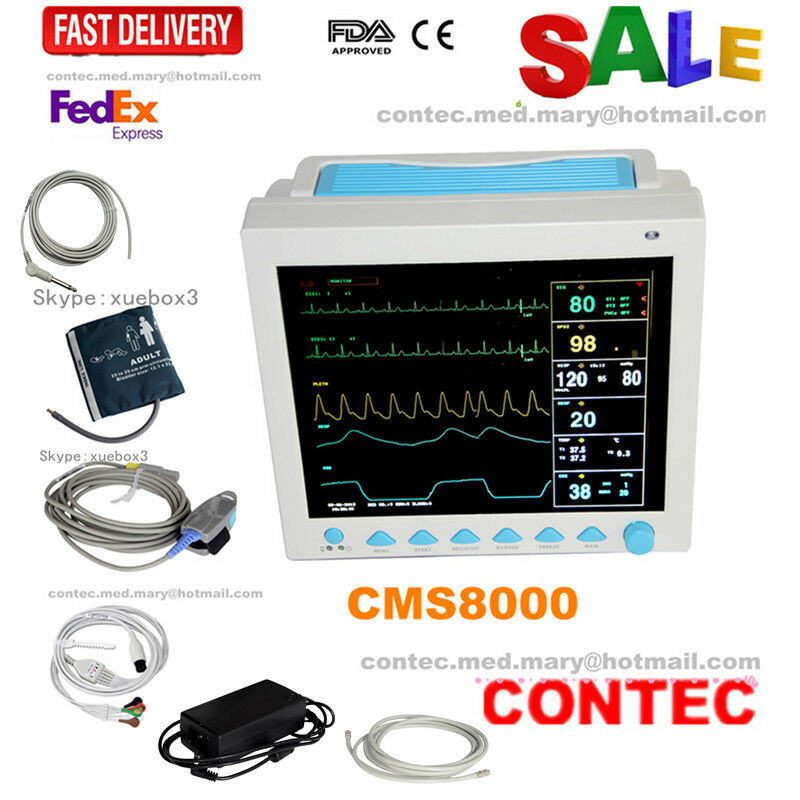
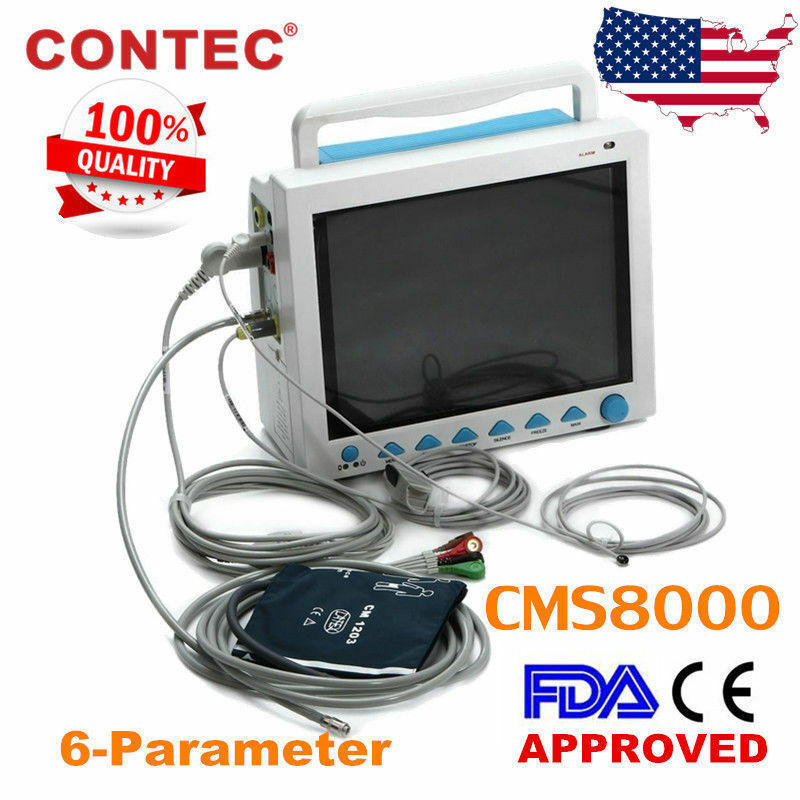
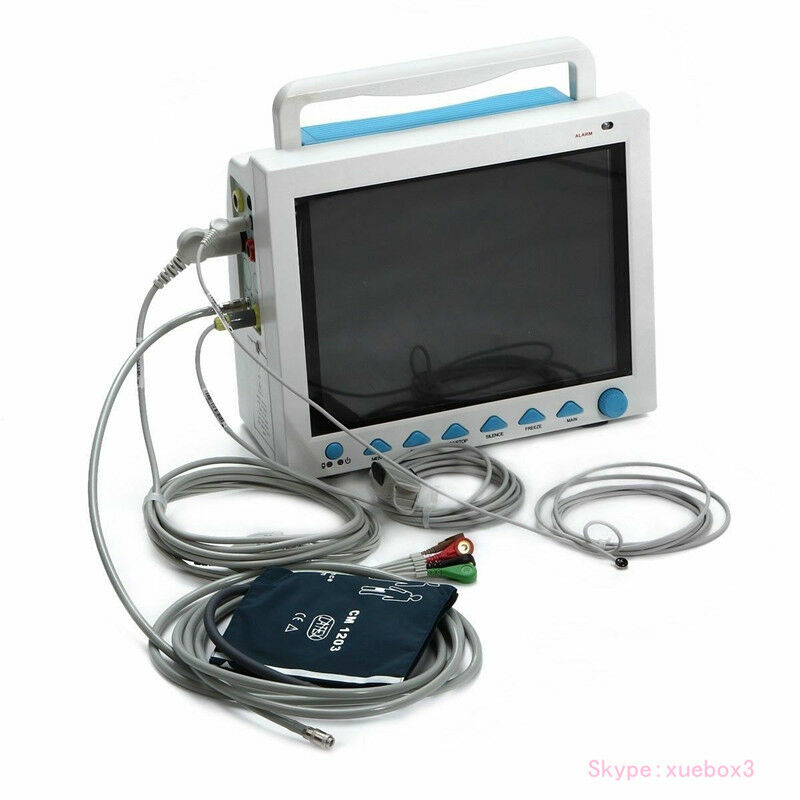
CONTEC FDA&CE ICU CCU Vital Signs Patient Monitor, 6 Parameters CMS8000 Newest
Introduction
The monitor has abundant functions that can be used for clinical monitoring with adult, pediatric and neonate. Users may select different parameter configuration according to different requirements. The monitor, power supplied by 100-240V~,50/60Hz, adopts 12.1'' color TFT LCD displaying real-time date and waveform. It can synchronously display eight-channel waveform and full monitoring parameters equipped with an optional 48mm thermal recorder. The monitor can be connected to the central monitoring system via wire or wireless network to form a network monitoring system.
This device can monitor such parameters as ECG, RESP, NIBP, SpO2, and dual-channel TEMP, etc. It integrates parameter measurement module, display and recorder in one device to form a compact and portable equipment. Its replaceable internal battery brings lot of convenient for patient moving.
Functions
Standard parameters: ECG, RESP, SpO2, PR, NIBP, dual-channel TEMP
1)ECG Heart rate(HR)
ECG waveform
Arrhythmia and ST-segment analysis
2)RESP Respiration rate(RR)
Respiration waveform
3)SpO2 Oxygen saturation(SpO2)
Plethysmogram(PLETH) waveform
Pulse rate(PR)
Bar graph
4)NIBP Systolic pressure(SYS), Diastolic pressure(DIA), Mean pressure(MEAN)
5)TEMP T1, T2, TD
6)IBP(optional) CH1:SYS,DIA,MAP
CH2:SYS,DIA,MAP
IBP waveform
7)CO2(optional) EtCO2
InsCO2:Inspired Minimum CO2
AwRR:Air Way Respiration
It has abundant functions, such as audible and visual alarm, trend data storage and output, NIBP measurement, alarm event marking and drug concentration calculation, etc.
Features
1)12.1'' TFT color LCD, multi-language interface(Simplified Chinese, Traditional Chinese, English, French, German, Turkish, Spanish, Portuguese, Italian, Dutch, Romanian, Russian, Kazakh, Polish, Czech).
2)Fanless design, quiet, energy-saving and clean, which reduces the possibility of cross-infection.
3)All-round monitor for adult, pediatric and neonate.
4)With standard interface, oxygen graph, trend graph, big character interface and view bed, convenient to observe.
5)Finish all operations by keys and knobs.
6)Maximum 8-channel waveform synchronous display.
7)Display 7-lead ECG waveform on one screen, cascade ECG waveform display
8)Adopt digital SpO2 technology, anti-motion and anti-ambient light interference, and measurement can be performed under the circumstance of weak filling.
9)Heart rate variability (HRV) analysis function
10)NIBP measurement mode: Manual/AUTO/STAT, storage for 4800-group NIBP data.
11)Review for 71 alarm events of all parameters and 60 arrhythmia alarm events.
12)Drug concentration calculation and titration table functions.
13)One-touch printing of trend graph
14)Connect to Central Monitoring System by 3G, Wi-Fi or wired mode.
15)AC/DC, built-in rechargeable lithium battery achieve uninterrupted monitoring.
16)Anti-high frequency surgical unit, defibrillation-proof (special leads are necessary).
Performance
▲ECG
Lead mode: 3-lead or 5-lead
Lead selection: I, II, III, aVR, aVL, aVF, V
Waveform: 5-lead, dual-channel
3-lead, single-channel
Gain: 2.5mm/mV, 5.0mm/mV, 10mm/mV, 20mm/mV, 40mm/mV
Scan speed: 12.5mm/s, 25 mm/s, 50 mm/s
HR:
Measurement and alarm range: 15~350 bpm
Accuracy: ±1 % or ±1 bpm, whichever is greater
Alarm accuracy: ± 2 bpm
Resolution: 1 bpm
ST-segment monitoring:
Measurement and alarm range: -2.0 mV ~ +2.0 mV
Accuracy: -0.8 mV~+0.8 mV ±0.04 mV or ±10%, whichever is greater
Other range: unspecified
Arrhythmia analysis: ASYSTOLE, VFIB/VTAC, COUPLET, BIGEMINY, TRIGEMINY, R ON T, VT>2,
PVC, TACHY, BRADY, MISSED BEATS, PNP, PNC
Pacemaker: yes
▲RESP
Method: R-F(RA-LL) Impedance
Respiration rate:
Measurement and alarm range: 0~150 rpm
Resolution: 1 rpm
Measurement accuracy: ±2 rpm
Alarm accuracy: ±3 rpm
Apnea alarm: 10~40s
Scan speed: 6.25 mm/s, 12.5 mm/s, 25 mm/s
▲NIBP
Method: Oscillometry
Mode: Manual/AUTO/STAT
Measurement interval in AUTO mode: 1/2/3/4/5/10/15/30/60/90/120/240/480/960 minutes
Measurement period in STAT mode: 5 minutes
Measurement and alarm range: 10 ~ 270 mmHg
Resolution: 1 mmHg
Cuff pressure accuracy: ±3 mmHg
Measurement accuracy:
Maximal mean deviation: ±5 mmHg
Maximal standard deviation: 8 mmHg
Over-pressure protection:
Adult mode: 297±3 mmHg
Pediatric mode: 240±3 mmHg
Neonatal mode: 147±3 mmHg
▲SpO2
Measurement and alarm range: 0 ~ 100%
Resolution: 1%
Measurement accuracy: 70%~100%: ±2%;
0%~69%: unspecified
▲PR
Measurement and alarm range: 30 ~ 250 bpm
Measurement accuracy: ±2 bpm or ±2%, whichever is greater
▲TEMP
Channel: dual-channel
Measurement and alarm range: 0 ~ 50℃
Resolution: 0.1 ℃
Accuracy: ±0.1 ℃
▲EtCO2(Optional function)
Method: Sidestream or Mainstream
Measuring Range: 0~150mmHg
Resolution:
0~69 mmHg, 0.1 mmHg
70~150 mmHg, 0.25 mmHg
Accuracy:
0~40 mm Hg ±2 mm Hg
41~70 mm Hg ±5%
71~100 mm Hg ±8%
101~150 mm Hg ±10%
AwRR Range: 2~150 rpm
AwRR Accuracy: ±1BPM
Apnea Alarm: Yes
▲IBP
(Optional function)
Channel: dual-channel
Label: ART, PA, CVP, RAP, LAP, ICP, P1, P2
Measuring and Alarm Range: -10~300 mmHg
Resolution 1 mmHg
Accuracy: ±2% or 1mmHg, whichever is greater
▲Power supply:
100-240V~, 50/60Hz
▲Safety classification: Class I, type CF defibrillation-proof applied part
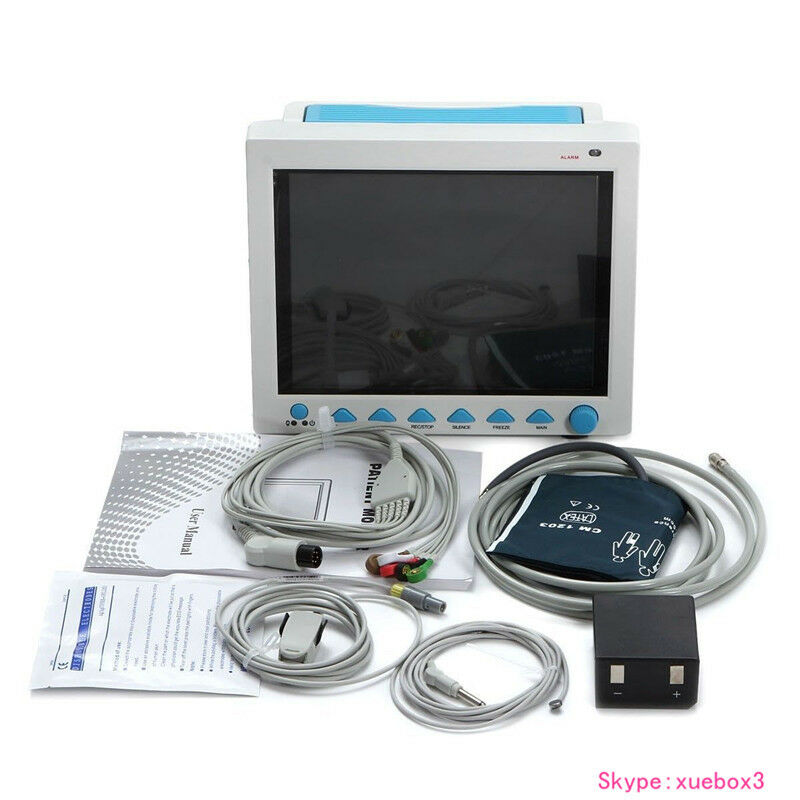
Accessories
1)Adult fingertip SpO2 probe (5-pin)
2)Adult NIBP cuff
3)NIBP extension tube
4)ECG lead cable
5)ECG electrode
6)Temperature probe
7)Power cord
8)User Manual
Physical characteristic
Dimension: 314 mm(L) × 145 mm(W) × 264 mm(H)
Weight: 3.9 Kg
Buy safe products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, we will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE,TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved
International Buyer--Please note:
Import duties,Taxes are not included in the item price.These charges are buyers' responsibility
We are the manufacturer of medical equipment for 17 years, the following are our main products:
ECG / ECG holter EEG / EEG Holter B-ultrasound
Patient Monitor Fetal doppler Fetal Monitor
Spo2 Monitor Stethoscope Medical Image....
Agent in the world!
We are look for partners in the world now!
Do you want to become our agent in you conutry?
Join US!
Skype:xuebox3
contec.med.mary at hotmail.com
Only English User Manual,Please contact us if you need other language version.thank you